2.1 Introduction
Rising fuel prices and the location of much of the petroleum reserves has led the United States and other countries to aggressively pursue the development of new technology for the conversion of renewable feedstocks such as wood, agricultural crops, municipal water, and animal residue to renewable fuels and chemicals. The challenges are enormous. It has taken decades to develop technology for the conversion of crude oil and gas into transportation fuels and chemicals that are abundant in our society. The transformation of petroleum to fuels and chemicals typically involves the cracking of carbon-carbon bonds using heterogeneous acid catalysts and hydrotreatment. However, renewable feedstocks require a new collection and distribution system and the conversion of wet, highly functional carbohydrates into products that will ideally be compatible with existing refinery operations. As a result, new catalysis and biology is being developed to convert these oxygenated feedstocks to useful products.
While governments have spent billions of dollars on research, development and piloting to encourage and assist in the commercialization of new processes, much innovation has come from small companies whose technology was often discovered in university research laboratories. These companies were founded with the financial assistance of venture capitalists and will hopefully be successful and develop into large and profitable enterprises. However, there is no intention to diminish the large R&D efforts by many companies such as Shell, BP, DuPont, Dow and others. This paper will highlight only a few of the small startup companies that are moving ahead rapidly as examples of the exciting new developments taking place in the renewable fuels and chemicals area.
2.2 Results and Discussion
Each year, Biofuels Digest polls a large number of readers and comes up with a list of the Top 50 companies that are active in the renewable fuels and chemicals area. A few of the Top 50 Bioenergy Companies for the 2010–2011 survey are shown in Table 2.1. Several of these will be discussed in this chapter. It is interesting to note the distribution of companies and their main focus. The feedstocks are diverse, the products are diverse and over 70% of the ranked companies are in the United States.
| Ranking | Company | Ranking | Company |
|---|---|---|---|
| 1 | Amyris | 11 | Virent |
| 2 | Solazyme | 12 | Mascoma |
| 3 | POET | 13 | Ceres |
| 4 | LS9 | 14 | Cobalt Technologies |
| 5 | Gevo | 15 | UOP |
| 6 | DuPont Danisco | 16 | Enerchem |
| 7 | Novazymes | 17 | BP Biofuels |
| 8 | Coskata | 18 | Genencor |
| 9 | Codexis | 19 | Petrobras |
| 10 | Sapphire Energy | 20 | Abengoa |
| 31 | LanzaTech | 47 | KiOR |
Tab. 2.1: A Partial Ranking of the Top Bioenergy Companies by Biofuels Digest (2010–2011) [1]. The complete ranking comprises 37 US and 13 non-US companies, 15 work in the field of cellulosic ethanol development, 5 with algae, 16 on renewable drop-in hydrocarbon fuels and 13 on renewable chemicals.
Tab. 2.1: A Partial Ranking of the Top Bioenergy Companies by Biofuels Digest (2010–2011) [1]. The complete ranking comprises 37 US and 13 non-US companies, 15 work in the field of cellulosic ethanol development, 5 with algae, 16 on renewable drop-in hydrocarbon fuels and 13 on renewable chemicals.
2.2.1 Renewable Fuels
The eventual transition from petroleum-based transportation fuels to renewable fuels provides a huge 200 billion gallon per year (1 trillion lbs) opportunity for new catalysis and process research. The renewable gasoline market is entirely dominated by ethanol, which is obtained by the fermentation of cornstarch as a necessary first step. New fuels and technology based on the use of non-food feedstocks are being developed and will hopefully be commercialized. It is recognized that the use of food feedstocks is not desirable in the long run but it is also important to recognize that the technical challenges for the conversion of carbohydrates are significant even with pure sugars such as glucose. Once that technology is developed new methods will be required to use real sugar solutions derived from lignocellulosic feedstocks. The nature of these feedstocks is illustrated in Figure 2.1. It is readily apparent that the real feedstocks which will contain biomass residues, tars, oligomers, inorganic metals from nutrient solutions, and sulfur, nitrogen and protein from fermentation derived feedstocks will pose significant challenges to maintained high conversion, selectivity and lifetime of new catalyst systems.

Fig. 2.1: Photos of Solutions Derived from Lignocellulosic Biomass and Pure Sugar (Photographs by courtesy of Dr. Edward J. Wolfrum, NREL)
Ethanol is widely used as an additive for gasoline. However, it has many limitations such as low energy density and cannot be transported in existing pipelines.
Bio-butanol is an attractive alternative to ethanol. A number of companies are actively working on organisms that will convert sugars to butanol. The benefits of butanol over ethanol as a fuel are shown in Table 2.2. Butanol offers better safety, improved fuel economy, can be blended with gasoline in high concentrations and used without vehicle modifications. Another important advantage is that butanol can be transported in existing pipelines.
| Property Comparison | EtOH | BuOH | Gasoline |
|---|---|---|---|
| Energy Content (BTU/gal) | 78M | 110M | 115M |
| Reid V.P. @ 100°F (psi) | 2.0 | 0.33 | 4.5 |
| Motor Octane | 92 | 94 | 96 |
| Air-to-Fuel Ratio | 9 | 11 | 12–15 |
Tab. 2.2: Comparison of ethanol, butanol and gasoline as fuels
Tab. 2.2: Comparison of ethanol, butanol and gasoline as fuels
| Company | Biofuel |
|---|---|
| Butamax (BP/DuPont) | iso-butanol n-butanol 2-butanol |
| Gevo | iso-butanol |
| Metabolic Explorer | n-butanol |
| Cobalt Biofuels | n-butanol |
| Green Biologics Ltd. | n-butanol |
| Tetravitae Bioscience | n-butanol (ABE) |
| Butalco | n-butanol |
Tab. 2.3: Non-limiting list of biofuel companies
Tab. 2.3: Non-limiting list of biofuel companies
A non-limiting list of companies that are active in this field is shown in Table 2.3. Large companies like BP and DuPont are moving rapidly to introduce butanol. Smaller startup companies like Gevo and Cobalt Biofuels have received significant venture capital funding and are making great progress with demonstrations in large pilot plants. Gevo Inc. was founded in 2005 with technology discovered at the California Institute of Technology. With some initial funding from Khosla Ventures, the company rapidly grew and after an IPO in February 2011, it currently has a valuation of nearly $500 million.
Gevo’s business model is to develop fermentation processes that can be retro-fit into existing ethanol plants with a limited amount of capital. Since 2009, Gevo is collaborating with ICM, one of the leading providers of ethanol production technology. They have successfully retrofit an existing ethanol plant at St. Joseph, Missouri, with an organism capable of making isobutanol and are currently in the process of retrofitting two additional ethanol plants to make isobutanol. The startup of the first plant is due in 2012. The current capacity is about 1 million gallons per year. They claim to be able to convert current ethanol plants to butanol production at relatively low cost. Of the three butanols, isobutanol is particularly attractive since it can be readily dehydrated to isobutylene, a valuable feedstock that is used in large volumes today. A biobased source of isobutylene provides an immediate opportunity to substitute a renewable product into existing investments. Some of the very large markets targeted by Gevo are shown in Figure 2.2. These cover a wide range of renewable chemicals and renewable fuels such as jet fuel, high-octane gasoline, solvents, renewable terephthalic acid for PET bottles and butyl rubbers.
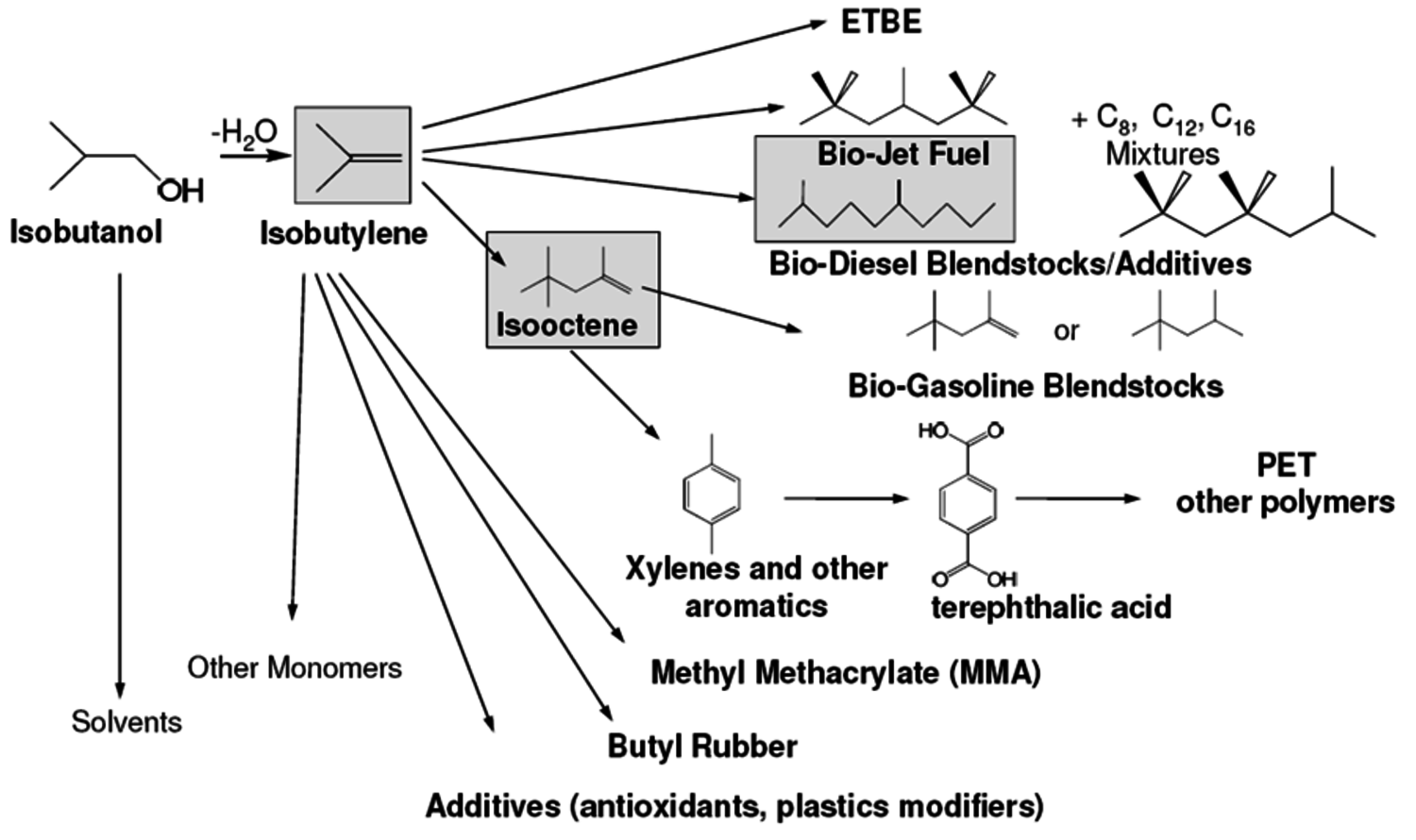
Fig. 2.2: Some Derivatives Available from Bio-Isobutanol
Amyris, Inc. is another successful startup that was founded with technology developed at UC Berkeley. Once again, with initial venture capital funding it grew rapidly and after an IPO in September 2010 the current valuation is over $ one billion. Their product platform is based on sugar fermentation to the isoprene trimer, β-farnesene (Figure 2.3). An attractive feature of their process is that the hydrocarbon product is insoluble in the fermentation broth and readily separates to provide the unsaturated C-15 molecule. After hydrogenation, it yields an attractive diesel fuel. In addition, the unsaturation provides a route to a variety of monomers, polymers and specialty chemicals.
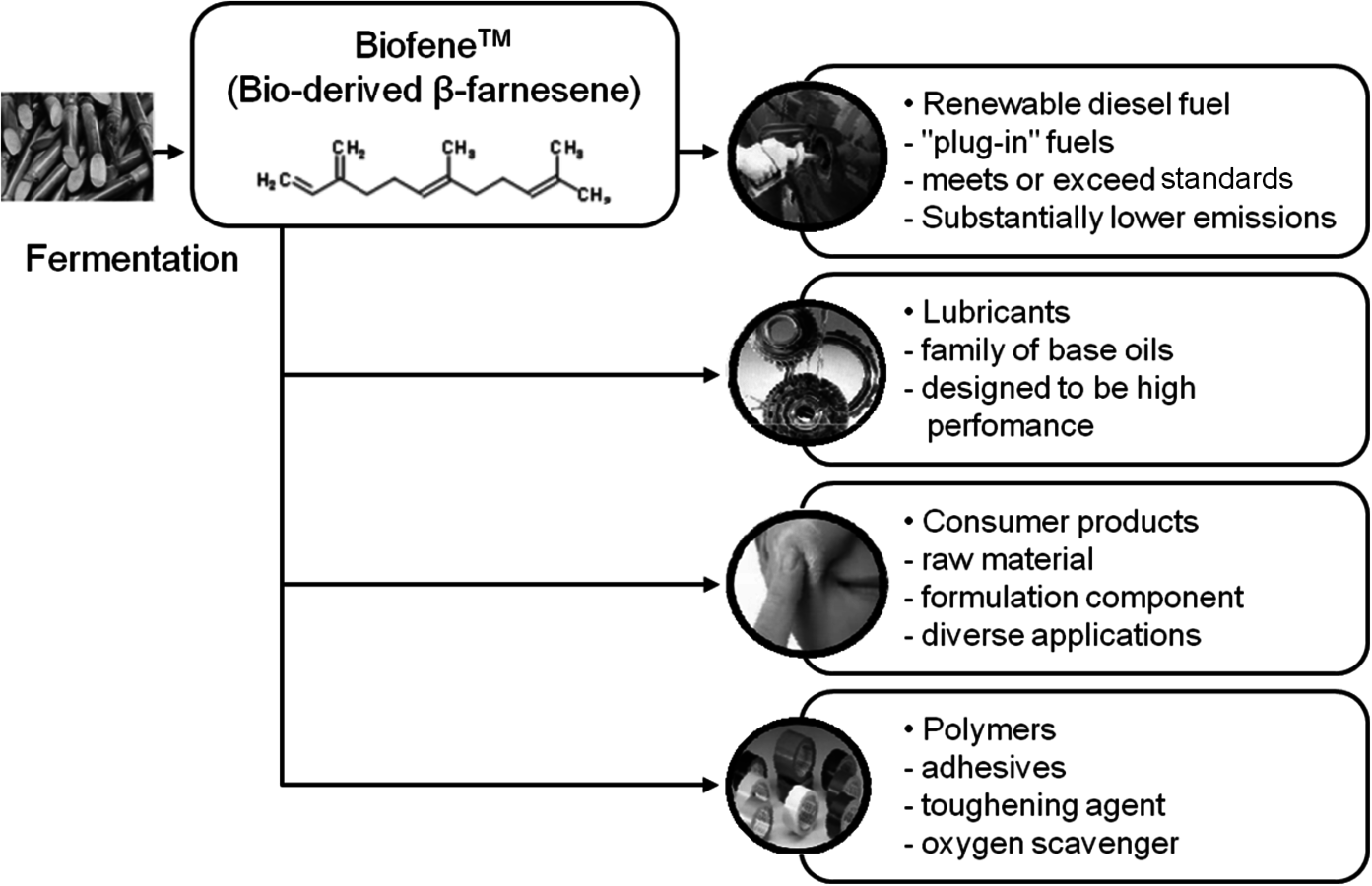
Fig. 2.3: Family of Products Derived from Amyris Biofene™
Another bio-company, which converts sugar to hydrocarbon products that readily separate from the fermentation broth, is LS9, Inc. Founded in 2005, it has developed a biological process for the fermentation of sugars to linear hydrocarbons as shown in Figure 2.4. By genetically altering the organism that naturally produces lipids, the company is able to make a variety of linear hydrocarbons that may be functionalized to alcohols, olefins, ketones and aldehydes. When this text was published, LS9, Inc. was still a private company.
LanzaTech Inc. is a startup company that was founded in 2005 in New Zealand. LanzaTech’s commercial plants effectively convert a variety of nonfood, low value gas feedstocks into bioethanol and other platform chemicals. Using proprietary technology they are able to ferment CO-rich streams into ethanol and other products like 2,3-Butanediol (Figure 2.5). The initial focus is on the conversion of waste streams from steel mills and gasifiers. They have been operating a pilot plant at a steel mill in New Zealand and have just announced the construction of a 100,000 gallon per year demo plant in collaboration with Bao Steel in China. The startup is expected in the first half of 2012. Following a successful scale up of their demo facility, commercial plants are planned for China, South Korea and India.
Next generation fuels, beyond ethanol and butanol derived from sugars, will be hydrocarbon fuels from lignocellulosic feedstocks. Direct gasification of bio-
mass to synthesis gas is technically feasible but presents a number of challenges such as the removal of tars and inorganics, which are severe poisons to downstream Fischer-Tropsch or higher alcohol synthesis catalysts [2].
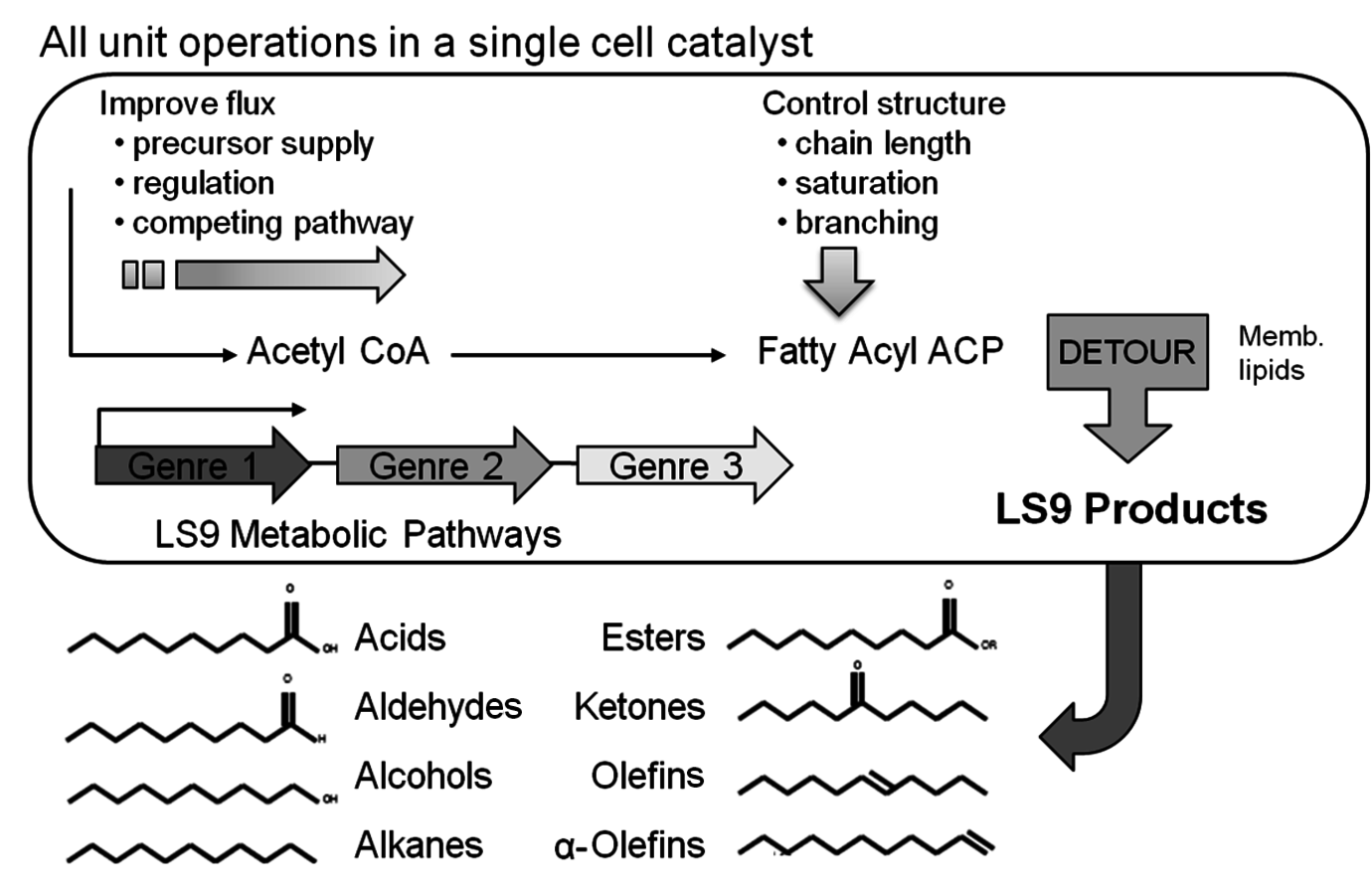
Fig. 2.4: Potential Biobased Products Produced by LS9, Inc.
An excellent overview of potential processes to convert lignocellulosic feedstocks to advanced biofuels may be found in a report from a U.S. Department of Energy (DOE) workshop in 2007 [3]. Biomass gasification potentially provides an excellent method of converting lignocellulosic feedstock into synthesis gas. New gasifiers are under development around the world since the variable composition of biomass is significantly different from natural gas or coal, the traditional sources of syngas. The inorganic content of some biomass can contain high levels of silica and other oxides as well as sulfur, nitrogen and phosphorus compounds, which are poisons for the downstream syngas conversion units. Not all startups are successful, unfortunately. Range Fuels, a privately held company in Colorado, received a loan guarantee from the DOE and raised over $100 million to build a demo plant in Georgia to gasify wood chips to syngas followed by an alcohol synthesis catalyst that would make primarily methanol and ethanol. Unfortunately, this plant did not work as expected. It was shut down and the staff of Range Fuels
dismissed [4].
Other processes of significant interest include:
1Conversion of municipal solid waste to ethanol or butanol using concentrated sulfuric acid (Bluefire Ethanol, Inc.)
2Gasification of biomass to syngas followed by the fermentation of the syngas directly to ethanol (Coskata, Inc.)
3Fermentation of sugars to 1,4-Butanediol (Genomatica, Inc.)
4Conversion of sugars to ethers of hydroxymethylfurfural (HMF) (Avantium – Netherlands)
5Hydrogenation of fats and oils to long chain linear alkanes for diesel fuel (Neste, Petrobras, ConocoPhillips, UOP, and others)
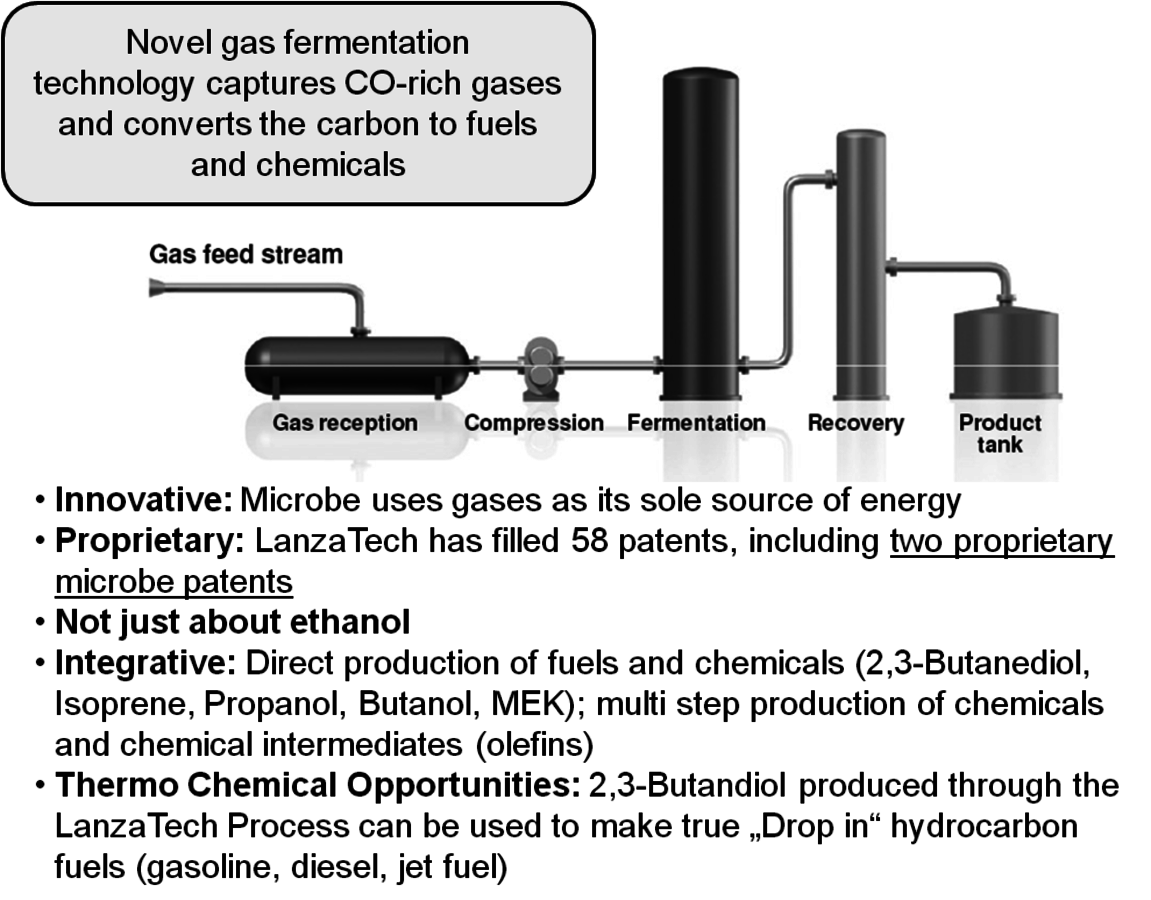
Fig. 2.5: The LanzaTech Process for Converting CO-rich Streams to Ethanol and 2,3-Butanediol
All of these new processes ultimately will require a large source of biomass to make a significant contribution to society’s need for transportation fuels. A 2005 report from the U.S. Department of Agriculture (USDA) [5] suggested that the U.S. had about 1.366 billion tons of agricultural and forest residue available but that this amount of biomass would satisfy only about 30% of the U.S. transportation fuel needs. Biomass has a high water content which is why economical transportation distances are usually considered about 50 miles or less. Much of the biomass considered in the USDA report is located at a significant distance from any potential biorefinery so it must be converted into a form that may be transported economically. One concept under consideration by many companies is field densification, or partial dehydration of the lignocellulosic material. A preferred process called fast pyrolysis involves heating the biomass very quickly (~1000 °C/sec) to a temperature of about 400 °C. During this heating step volatile gases are produced, along with a solid char (from the lignin) and a wet oil called pyrolysis oil. The oil tends to be very acidic and corrosive, highly colored, immiscible with hydrocarbon fuels and contains about 20–30% residual water. It also is relatively unstable and its composition changes with time. The char and the volatile gases may be burned to provide the heat needed for the process.
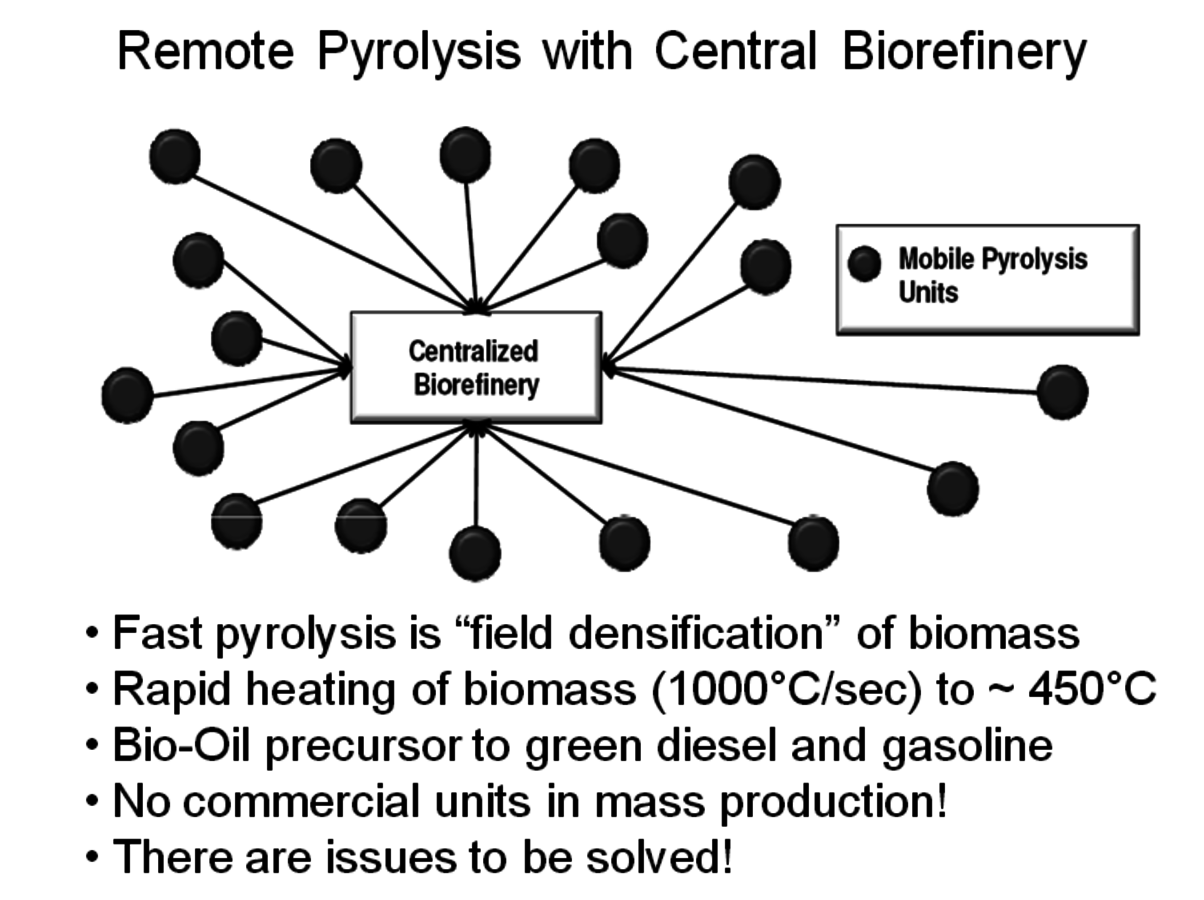
Fig. 2.6: A Concept for the Collection of Remote Biomass
A number of companies are working on modular fast pyrolysis units that will fit on the bed of a truck and can be moved from site to site as needed (Figure 2.6). Stabilizing and upgrading of the pyrolysis oil prior to shipment is one of the many challenges to commercial use. Ideally, this treatment will significantly deoxygenate the pyrolysis oil so that it becomes less water soluble and phase separates from the water. UOP and Ensyn have formed a Joint Venture called Envergent Inc. with the purpose of developing technology to hydrogenate the pyrolysis oil to a useful hydrocarbon fuel (Figure 2.7). Their current process uses a circulating solids reactor with sand as the heat transfer media.

Fig. 2.7: Envergent, a Joint Venture between UOP and Ensyn
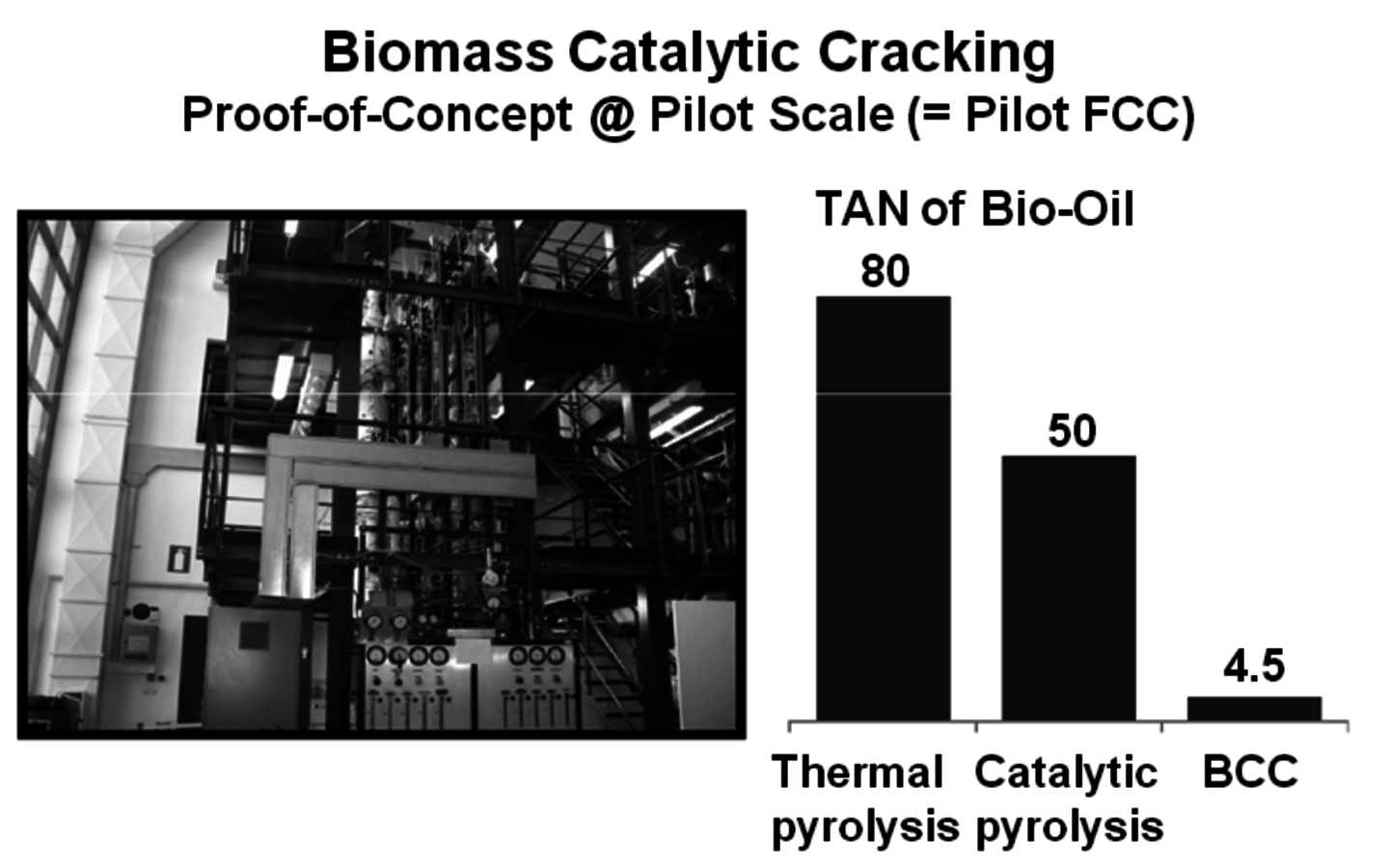
Woody biomass is brought in contact with the hot sand at about 510 °C for two seconds to produce pyrolysis oil (Figure 2.8). KiOR Inc. is another startup company based in Texas. A significant difference in their process compared to others is that a catalyst is used during the pyrolysis step. The effect is very significant since the catalyst allows the biomass to be pyrolyzed at a lower temperature while at the same time reducing the oxygen content of the pyrolysis oil. The Biomass Catalytic Cracking process (BCC) (Figure 2.9) produces oil with a Total Acid Number (TAN) much lower than that of non-catalytic processes. As a result, a hydrocarbon phase separates and can be readily processed. Dynamotive is another fast pyrolysis company that has several commercial plants making pyrolysis oil for energy use. It is developing a two-stage hydrogenation process that first stabilizes the oil by reducing the water and oxygen content and then hydrotreats the first product to give a gasoline/diesel fuel.
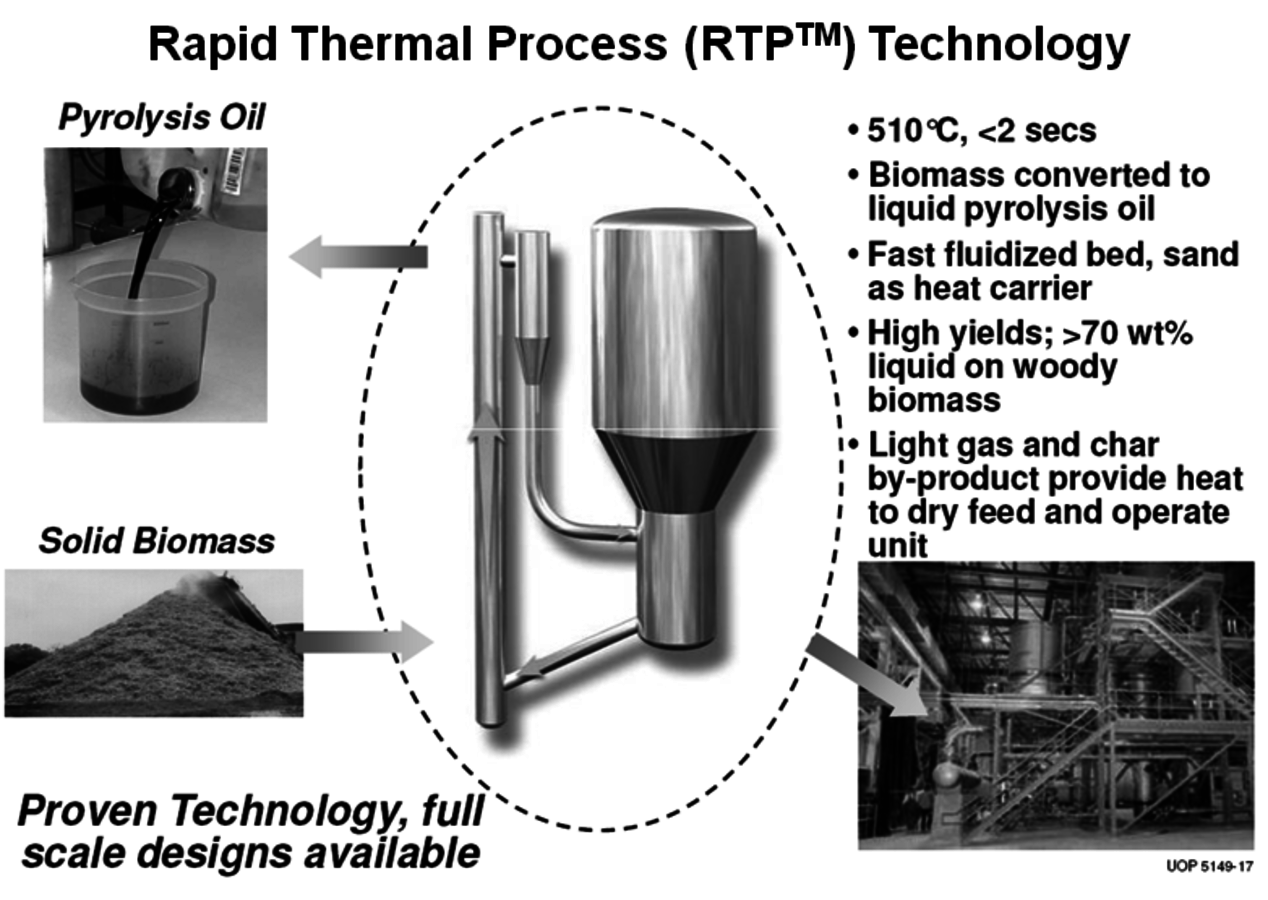
Fig. 2.8: Envergent’s Process for the Production of Pyrolysis Oil
2.2.2 Renewable Chemicals
Chemicals are higher value products than fuels. For this reason a number of companies are working on biological and thermochemical routes to renewable chemicals. In some cases, the products are direct drop-in molecules while in other cases new products are obtained. An important and commonly cited report was prepared by PNNL and NREL [6]. After considerable study, they proposed twelve molecules, shown in Table 2.4, that would be attractive building blocks for future renewable chemicals. This report has stimulated a very significant amount of research within the academic and industrial communities.
| 1,4 diacids (succinic, fumaric and malic) | itaconic acid |
| 2,5-furandicarboxylic acid | levulinic acid |
| 3-hydroxypropionic acid | 3-hydroxybutylrolactone |
| aspartic acid | glycerol |
| glucaric acid | sorbitol |
| glutamic acid | xylitol/arabinitol |
Tab. 2.4: Top 12 Sugar-Derived Building Blocks
Tab. 2.4: Top 12 Sugar-Derived Building Blocks
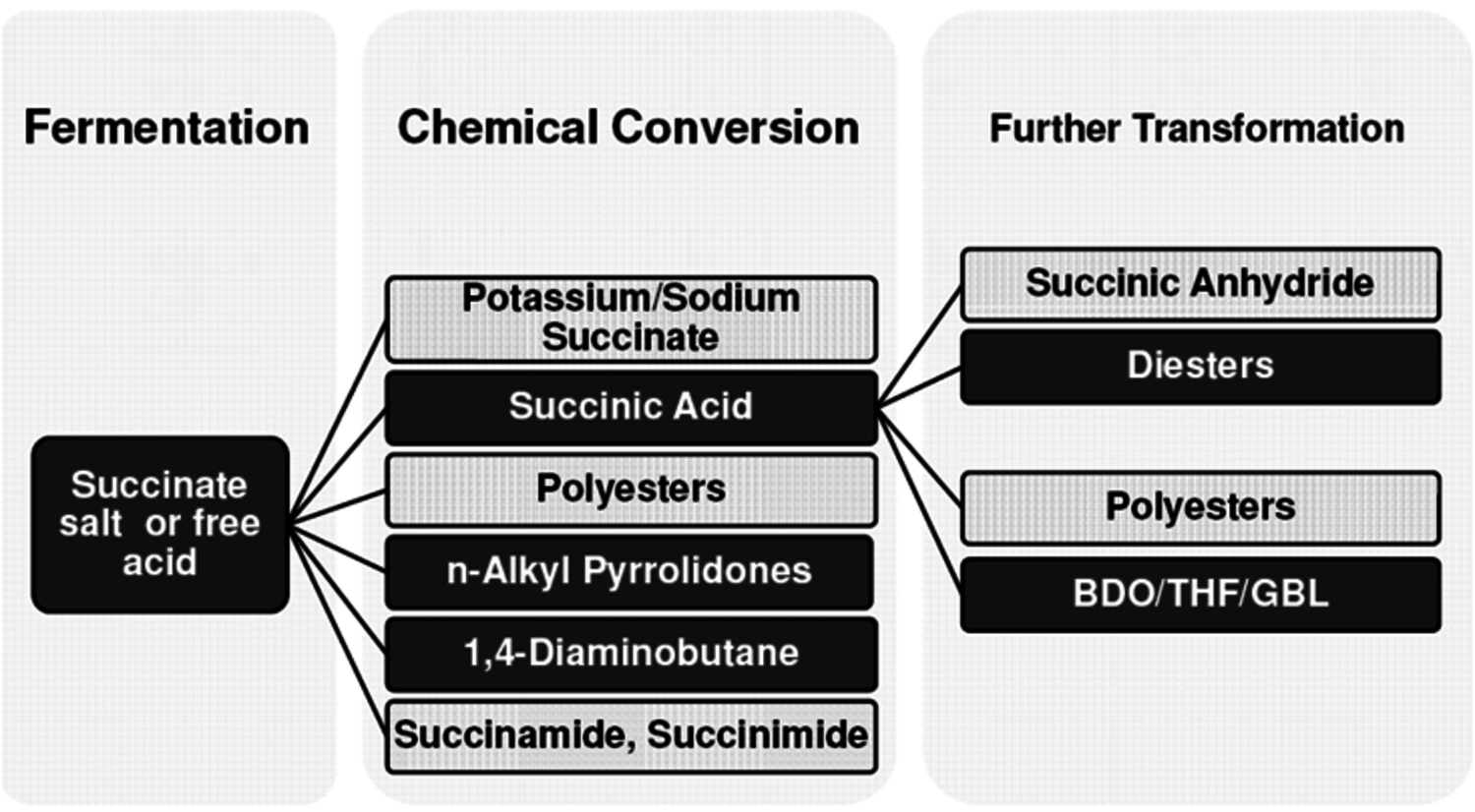
One molecule that is receiving considerable attention is succinic acid. A number of small and large companies (BioAmber, Myriant, DSM, BASF and others) have announced plans to commercialize bio-based succinic acid and its derivatives. BioAmber Inc. commissioned the world’s first commercial plant in France in January 2010 with an initial capacity of 2,200 MT per year. Succinic acid is a particularly attractive molecule as it is produced today from maleic acid via butane oxidation. A biobased source of succinic acid and its derivatives would be drop-in replacements for the petroleum-derived compound. The markets for products derived from bio-based succinic acid (polymers, plastics, solvents, adhesives, and coatings) (Figure 2.10) are significant so it is not surprising that it has attracted the attention of so many companies.
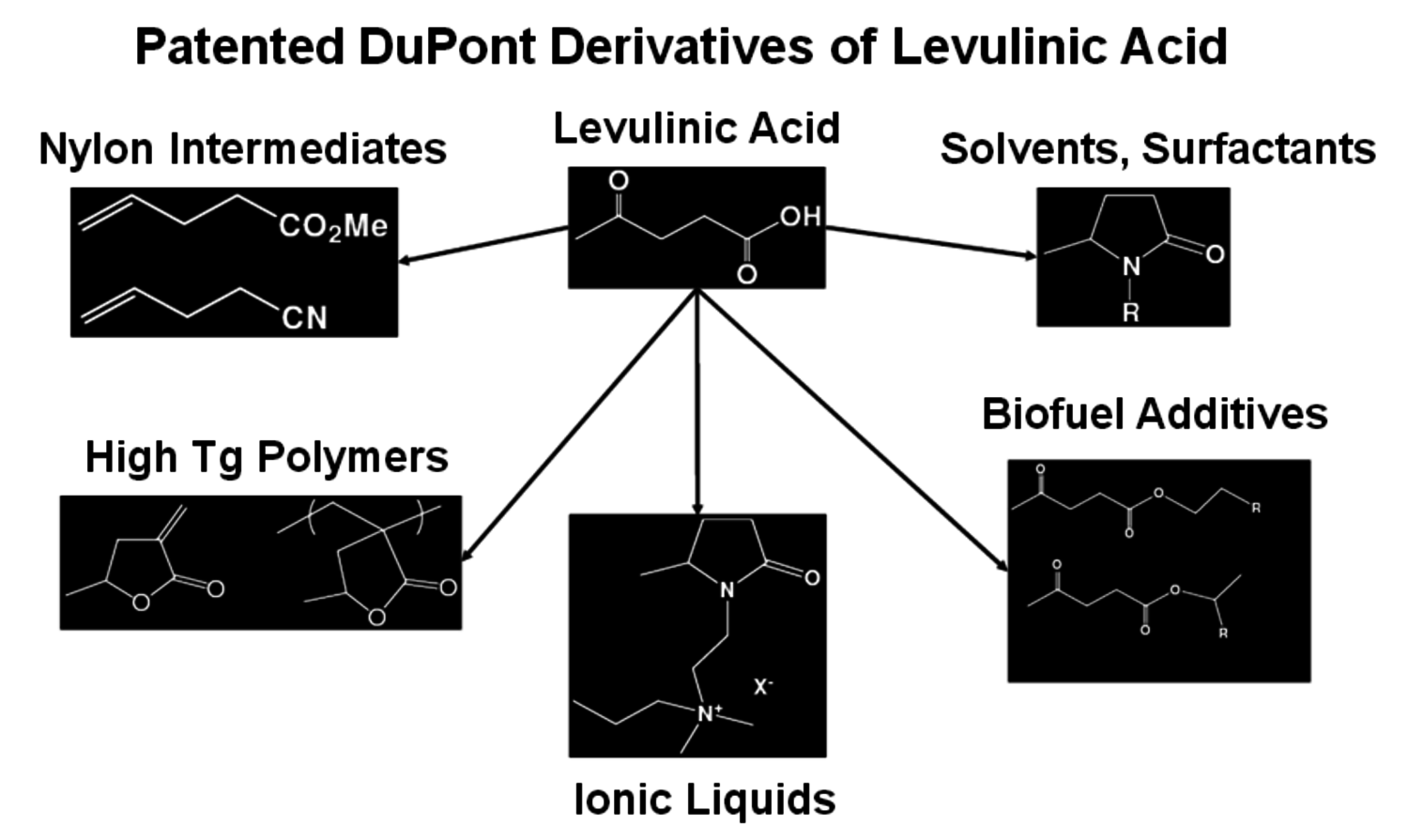
Levulinic acid has been of interest for many years as a bifunctional keto-acid but it has never been made on a commercial scale [7]. Biofine Renewables, LLC has developed a thermochemical process that produces levulinic acid, furfural, formic acid and char from a wide variety of biomass feedstocks and is operating a fully integrated pilot plant. The process uses two reactors to initially break down the biomass into small components such as hydroxymethylfurfural which are then further converted to levulinic acid. The conditions are extreme, with high temperatures and pressures but a yield of about 50% levulinic acid is achieved. With the ketone and acid group functionality, levulinic acid can be converted to a wide variety of compounds such as N-alkylpyrrolidones, monomers for the preparation of thermally stable polymers, ionic liquids and nylon intermediates (Figure 2.11).
While many of these materials are exciting new renewable based chemicals, none have reached commercial production due to the fact that a low cost, high volume source of levulinic acid is not yet available. Some other derivatives of levulinic acid have reached advanced stages of development and are moving to advanced production levels. One example is the startup company Segetis, Inc. It initially received venture capital funding to develop a novel class of ketals formed by the reaction of diols with the ketone group of levulinic acid. Using glycerol and levulinic acid, two of the DOE “Top 12” molecules, new ketal products are produced which have excellent functionality and can potentially replace existing petroleum based solvents, surfactants and plasticizers (Figure 2.12).
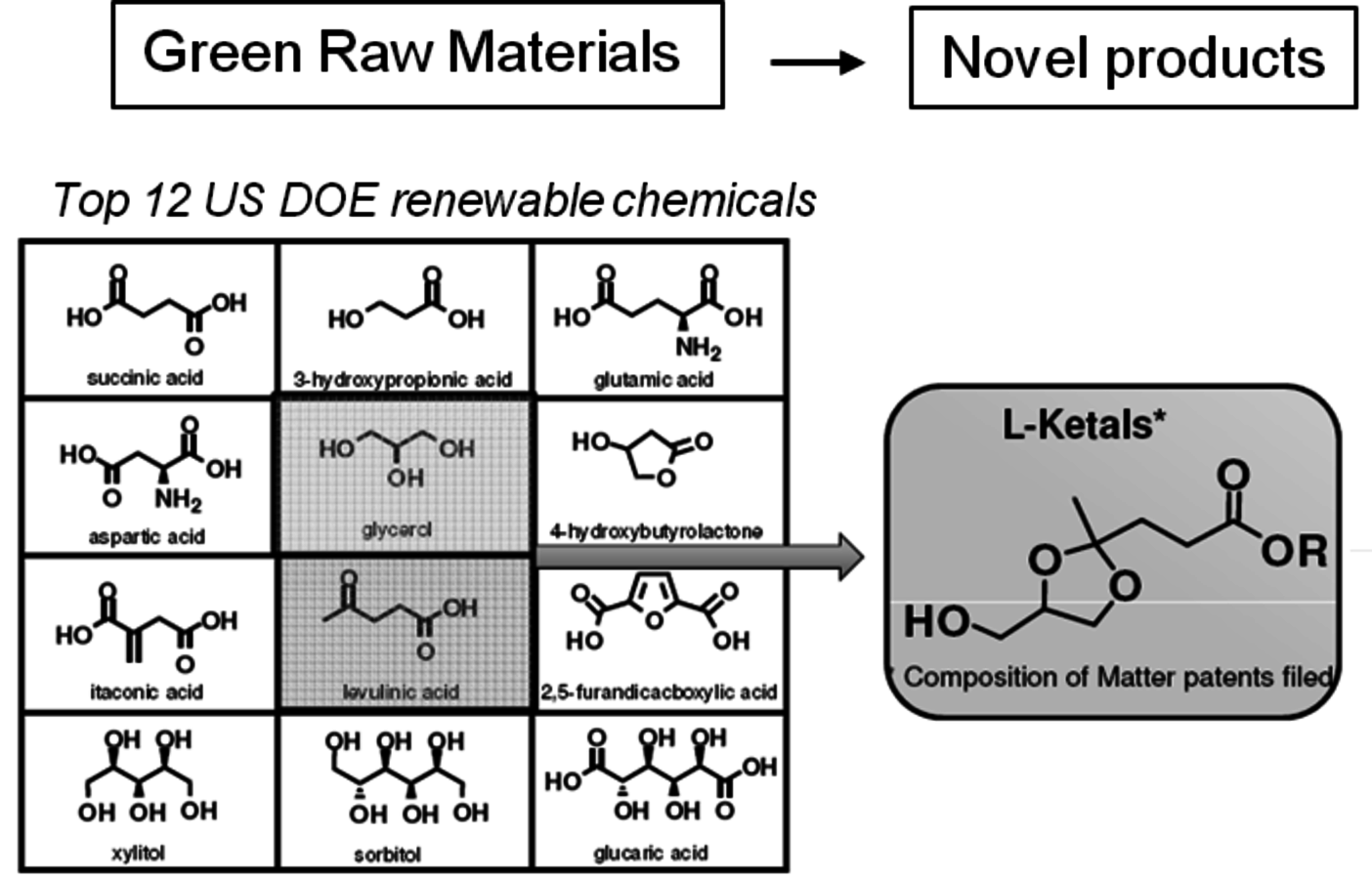
Fig. 2.12: Segetis Ketals are Derived from Diols and Levulinic Acid. Levulinic Ketal esters can be readily extended with alcohols, esters, and amines using standard catalysts and reaction conditions to produce a wide range of bio-based ester technologies.
Furandicarboxylic acid (FDCA) is another one of the DOE “Top 12” mole- cules. It may be prepared by oxidation of hydroxymethylfurfural, HMF (Figure 2.13), which is a difficult molecule to obtain due to its high reactivity. Avantium is pursuing the use of HMF-ethers as renewable diesel compositions. An advantage of forming the HMF-ether is that it is much more stable and easier to work with than HMF. Polymers of FDCA [8], are potential replacements for terephthalic acid esters (Figure 2.14). However, there has never been a viable process for its manufacture. The HMF ethers can provide an excellent feedstock to FDCA by oxidation. Avantium has collaborated with Nature Works to explore the commercial potential of FDCA polyesters.
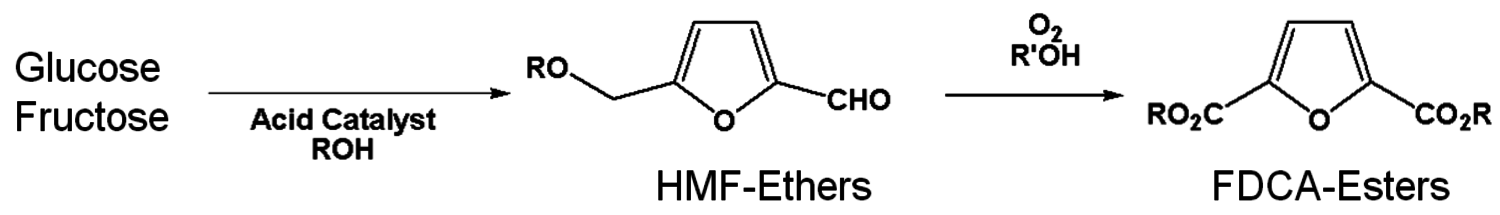
Fig. 2.13: Reaction scheme of C6 sugars to FDCA-esters
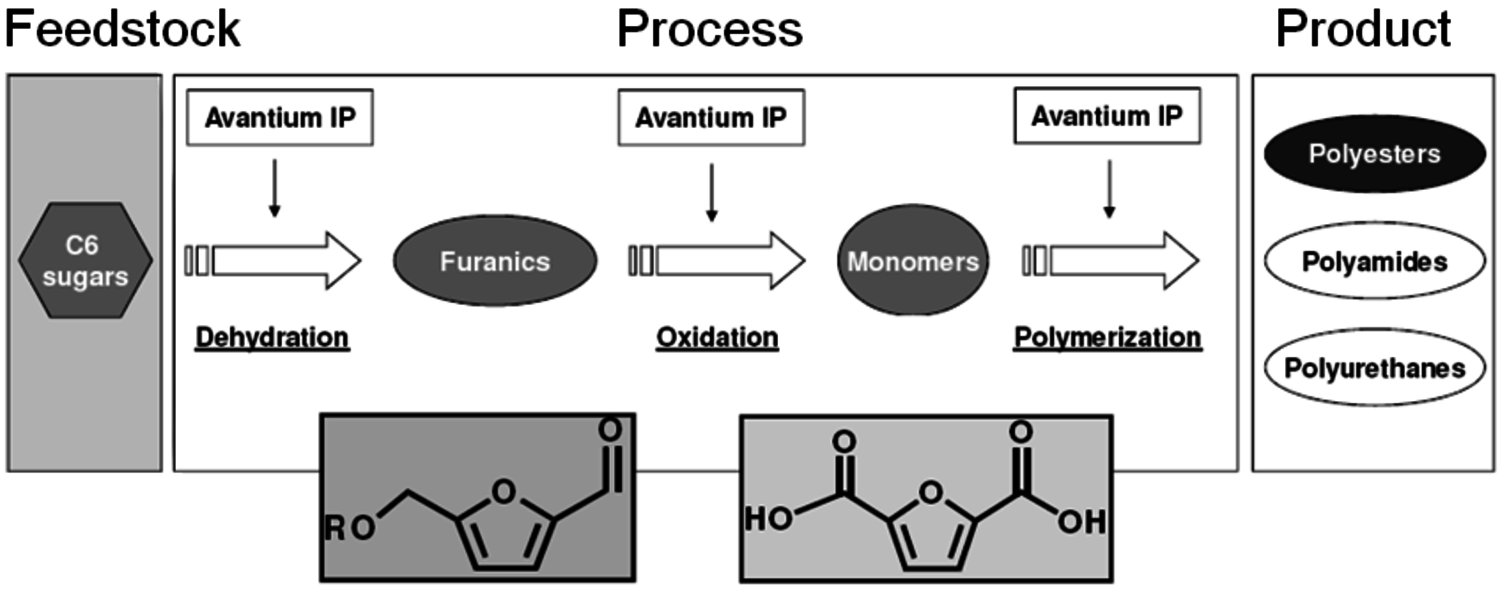
Fig. 2.14: Scheme of the Avantium process from C6 sugars to polymers
2.3 Conclusion
The intent of this chapter was to provide examples of the creativity and innovation being developed mostly by new startup companies. These companies are playing a key role in the development of renewable fuels and chemicals. Not all will be successful, larger oil and chemical companies will acquire some, and some will grow into new companies. With luck, perseverance and significant funding the world’s needs for fuels and chemicals will someday be based on renewable feedstock. In the 2+ years since this presentation, companies like Amyris, Gevo, KiOR and others have run into scale-up problems and delays resulting in significant decreases in their stock price and valuation since their initial IPO. Hopefully these problems will be overcome and they will be successful but only time will tell.
Acknowledgments
I wish to thank many individuals (too numerous to name) in a number of startup companies for providing me with the information discussed in this text and allowing it to be publically disclosed.
Bibliography
[1]
[2] S. Phillips, A. Aden, A. A., Jechura A. () Thermochemical Ethanol via Indirect Gasification and Mixed Alcohol Synthesis of Lignocellulosic Biomass.
[3] G.W. Huber. Breaking the Chemical and Engineering Barriers to Lignocellulosic Biofuels: Next Generation Hydrocarbon Biorefineries, National Science Foundation. Chemical, Bioengineering, Environmental, and Transport Systems Division. Washington, DC: NSF, 2008
[4] The Range Fuels Fiasco. The Range Fuels Fiasco Wall Street Journal (2010, February 10th):.
[5] USDA () Biomass as a Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply.
[6] T. Werpy, G. Petersen () Top Value Added Chemicals From Biomass.
[7] S. Ritter. Biorefinery Gets Ready to Deliver the Goods Chemical & Engineering News 84: 47 (2006): 47.
[8] A. Gandi, M.N. Belgacem. Furans in Polymer Chemistry Progress in Polymer Science 22: 1203-1379 (1997): 1203-1379.