Chapter structure
- 6.1 Introduction
- 6.2 Why Cellulosic Biomass?
- 6.3 Conversion Options for Aqueous Phase Processing
- 6.4 Laboratory Methods to Make Reactive Intermediates
- 6.5 Pretreatments and Biological Production of Sugars as Reactive
Intermediates Through the Consortium for
Applied Fundamentals and Innovation (CAFI) - 6.6 Thermochemical Processing to Sugars and
Other Reactive Intermediates - 6.7 Conclusions
- Bibliography
6.1 Introduction
Petroleum is the largest source of energy for the world, supplying about 1/3 of total world energy, and about 2/3 of world petroleum reserves are in the Mideast.
Furthermore, about 2/3 of petroleum goes to transportation which in turn relies on petroleum to provide about 97% of its energy. In addition, the transportation sector is a major source of greenhouse gases, contributing more than any other end user in the U.S. [1]. Thus, we need to find sustainable alternatives to petroleum for transportation to avoid future transitions and reduce greenhouse gas emissions.
Over the decades, there has been recurrent talk of reducing our dependence on petroleum. This plea started when the U.S. oil production peaked in 1970 at 9.6 million barrels per day, followed by the OPEC oil embargo of 1973 that created economic chaos. Not long after that, on April 18, 1977, President Carter declared the “moral equivalent of war” in developing new energy sources with the warning that “it will get worse every day until we act.” Virtually every president of the U.S. since that time has committed to reducing petroleum use. However, the real result is anything but convincing. For example, since 1973, the world consumed about 900 billion barrels of oil of the more than 1.1 trillion barrels used to date. In addition, world oil consumption has increased from 56.7 million barrels per day in 1974 to 84.6 million in 2006. For the U.S., consumption has risen to 5.1 million barrels per day. World petroleum reserves now stand at about 1.1 to 1.3 trillion barrels of oil including oil sands in Canada, a total that would last only about 40 years at current world consumption rates. Perhaps even more frightening, atmospheric carbon dioxide levels measured at Mauna Loa rose from about 330 ppm in 1974 to about 380 ppm in 2008, a 17% increase. Few measures have been taken to replace oil other than cane sugar ethanol in Brazil and the now much maligned corn ethanol industry in the U.S., even though they are effective in reducing the use of oil at least somewhat [1].
Faced with this gloomy forecast, what should we do? Some advocate “Drill baby, drill” as the answer, but in fact, U.S. energy reserves would only last a few years if we were to rely on them as our only resource. Although new oil is continually discovered, the rate of discovery is lower than the rate of consumption, making this a path of limited opportunity. Against that, we have three options. First, we could change the source of fuels to options such as coal. However, it is vital that the new resource be sustainable such as biomass to avoid GHG emissions and also avoid future transitions. The second option is to use more public transportation and drive less miles. This is an important opportunity but counters historic trends. Finally, we could drive more efficient vehicles, an option that is generally very feasible and synergistic to introducing new fuels that are not likely to be as cheap or as abundant as petroleum.
6.2 Why Cellulosic Biomass?
Petroleum is favored because it is a liquid with high energy density that can be rapidly replenished in vehicles. However, no other abundant resources are high energy content liquids that can be employed in this service. Abundant fossil options include natural gas and coal, but neither is as easily used as petroleum. Oil sands and shale are abundant fossil resources, with the former being now converted into liquid fuels in Canada. However, both have large environmental footprints in access and conversion. Furthermore, none of these fossil alternatives are sustainable and all will contribute greenhouse gas emissions that cause global climate change.
If we turn to sustainable resources, as we must sooner or later, we only have the choices of using the sun, wind, ocean waves, ocean temperature gradients, hydropower, geothermal energy, or nuclear power [2]. However, none of these options are liquids or for that matter lend themselves to mobile applications. Rather we must first capture each as stored energy. For example, all of these resources can be converted into electricity, but we must then store the electrical energy for transportation either by hydrolyzing water into hydrogen or charging batteries or other storage devices. Alternatively, plants can capture the sun’s energy directly by combining water and carbon dioxide through photosynthetic reactions to form biomass. Although biomass itself is a solid that would not lend itself to powering transportation, it can be converted into liquid fuels that are more than capable of powering all classes of vehicles with minimal changes in infrastructure, particularly compared to the major changes needed to accommodate a shift to batteries or hydrogen power. In fact, biomass is the only route to sustainable production of liquid transportation fuels [2]. Thus, while light duty cars and trucks may be able to use hydrogen or batteries if the required infrastructure changes can be made and consumers are willing to sacrifice the convenience of liquid fuels, heavy duty vehicles and aircraft will be forced to use biofuels to meet their needs sustainably.
Plants come in many shapes and sizes to meet many purposes. Some such as sugar cane and sugar beets are grown to take advantage of their production of sugars that are easily extracted for food uses. Others such as corn capture sugars in long chains as starch and can be used directly as animal feed or human food or readily broken down to their component sugars for use in soft drinks and many other foods. Plants also produce oils in their seeds that can be extracted for food or industrial uses. All three of these forms of solar energy storage in plants, that is, sugar, starch, and oils, can be readily used to produce transportation fuels, but none of them are available in anywhere near enough quantity to impact the vast transportation fuel market in a substantial way. Furthermore, because of their value as food, conversion to transportation fuels sparks controversies about direct and indirect competition with food production, limiting their long term prospects.
Plants also capture the sun’s energy in structural carbohydrates known as cellulose and hemicellulose that support plants. Cellulose is a long, linear chain of glucose sugar molecules that form tight hydrogen bonds with neighboring chains to form extensive crystalline regions that become the fibers in plants. Hemicellulose is also a sugar polymer but made up of up to the five sugars arabinose, galactose, glucose, mannose, and xylose as well as other molecules. These chains are branched and not crystalline but serve to glue cellulose chains together. A phenyl propene polymer known as lignin works with hemicellulose in this role, but lignin is not made of sugars. Rather, it resembles coal more closely than sugars. About 40 to 50% of typical plants such as wood, grasses, and agricultural residues is cellulose, another 20 to 30% is hemicellulose, and about 15 to 25% is lignin. Other components including free sugars, minerals, and oils make up the remaining portion, with the amounts depending on such factors as the plant type, harvest season, location, storage conditions, and climate.
Cellulosic biomass is attractive for making fuels because it is more abundant than other biomass types. For example, a recent USDA/DOE sponsored study predicted that well over 1 billion dry tons of biomass could be sustainably available in the long term for making fuels in the United States [3]. This quantity would be sufficient to possibly make enough fuel to displace about 80 billion gallons of gasoline compared to the current U.S. gasoline consumption of 140 billion gallons. Some profess to worry about the density of biomass and the resulting impact on transportation to central processing facilities. Yet, it is easy to show that if cellulosic biomass could be grown at a productivity of 10 dry tons/acre/year and we could realize yields of about 70 gallons of gasoline equivalent per dry ton, approximately 3.5 billion gallons of gasoline could be displaced in a 50 mile radius, which is a distance typically considered acceptable for moving corn or wood to existing central processing facilities for making corn ethanol and paper products, respectively. Even assuming that some of the land may not be available for growing energy crops or that yields may be lower, it is quite feasible to realize well over a billion gallons of gasoline equivalent within the 50 mile radius. Furthermore, cellulosic biomass is low in cost, with biomass costing $60 per dry ton equivalent to petroleum at $20 per barrel on an equal energy content basis [4]. Thus, the challenge is to convert this abundant, low cost resource into liquid transportation fuels at low costs.
6.3 Conversion Options for Aqueous Phase Processing
Although cellulosic biomass is a unique resource for large scale capture and storage of solar energy, it stores energy in a solid while we prefer the convenience of liquid and gaseous fuels, since they are much better suited to transportation applications that now consume much of the petroleum used. Furthermore, liquid fuels from biomass are the only known option for sustainable production of jet and diesel fuels and are virtually certain to have a vital role as we transition to sustainable energy sources. Thus, we must develop low cost processes to convert solid biomass into liquid fuels for transportation.
In simple terms, the composition of cellulosic biomass can be viewed as consisting of fixed carbon, volatile matter, ash, and moisture via what is often called proximate analysis. For example, switchgrass may have typical values of 17.1, 58.4, 4.6, and 20.0 wt%, respectively, and a lower heating value (LHV) of 13.6 MJ/kg and a higher heating value (HHV) of 15.0 MJ/kg. Such information may be sufficient if the intent is simply to burn the material. However, we can also obtain elemental compositions in what is typically designated as ultimate analysis of carbon, hydrogen, oxygen, nitrogen, sulfur, and ash contents, with possible values for switchgrass being 47.0, 5.3, 41.4, 0.5, 0.1, and 5.7 wt%, respectively, on a dry biomass basis. In this case, the LHV and HHV will be greater due to the lack of moisture, with values of 17.0 MJ/kg and 18.7 MJ/kg being representative. This information may be sufficient for thermal conversion approaches that focus on capture of the key elements as fuels. However, cellulosic biomass is made up of a complex network of long cellulose chains that are held together by hemicellulose, lignin and various other components to provide support and promote growth of plants. Cellulose is made up of long chains of covalently bound glucose sugars that are linked to adjacent cellulose chains by hydrogen bonding to form cellulose fibrils, with a large portion being crystalline. Hemicellulose is typically comprised of arabinose, galactose, glucose, mannose, and xylose sugars that are also bound to each other and smaller amounts of other compounds covalently. These compounds can be released from biomass by addition of one molecule of water to one molecule of the anhydrous sugars known as arabinan, galactan, glucan, mannan, and xylan to form the corresponding sugars in solution through a hydrolysis reaction. Lignin, on the other hand, is not a carbohydrate but is made up of phenyl-propene units. Lignin and hemicellulose work to hold the cellulose structure together in a strong composite material. As one example, switchgrass may contain about 35% glucan, most of it being in cellulose, about 21.8% xylan, 3.5% arabinan, 21.4% lignin, 3.3% ash, and 13.8% other compounds such as free sugars, protein, oils, and starch.
Huber et al. outlined in some detail the variety of routes by which cellulosic biomass can be converted into liquid fuels [5]. These can be divided into thermal routes and aqueous processing approaches. In simple terms, thermal routes involve breakdown of biomass at high temperatures into simple components that can then be recombined to form more complex fuel molecules or directed toward just hydrogen. One set of such thermal routes gasifies biomass to a mixture of carbon monoxide and hydrogen (syngas) that can be converted into diesel fuel via Fischer-Tropsch catalysis or other products including methanol and hydrogen. Other thermal routes employ liquefaction or pyrolysis to form bio-oils that can be upgraded to aromatics and other hydrocarbons by hydrodeoxygenation, zeolite catalysis, and other approaches.
Another set of options is built around aqueous phase processing of cellulosic biomass to release sugars or their dehydration products for subsequent biological or catalytic conversion to fuels. The intent of aqueous processing is to depolymerize biomass into its monomer units, thereby preserving much of the complex structure from which to build fuels. Thus, lower temperatures in the range of 140 to 220 °C are typically applied to avoid loss of these compounds during processing. The sugars that make up hemicellulose can be recovered with good yields of 85% and more by applying dilute sulfuric or other acids at 140 to 170 °C or higher. However, the crystalline structure of cellulose makes it challenging to recover glucose with yields over 60% via thermal routes, and cellulase enzymes are favored to catalyze breakdown of cellulose to glucose with high yields. Unfortunately, high enzyme costs stand in the way of low cost glucose from cellulosic biomass. Alternatively, arabinose and xylose in hemicellulose can be dehydrated to furfural by holding these sugars for longer times at high temperatures, although steps will be needed to achieve higher furfural yields than realized in commercial systems now. Holding reactions for longer times will dehydrate glucose to hydroxymethyl furfural (HMF) that in turn will break down to form levulinic and formic acids in equal molar quantities. It is difficult to capture HMF with high yields, but good yields of levulinic and formic acids can be achieved.
Enzymes are very selective catalysts for the breakdown of cellulosic biomass to form sugars. For example, in the case of cellulase enzymes which attack cellulose to release glucose sugar, these enzymes function as three primary components [6]. The first is called endoglucanase and attacks cellulase chains along their length to form ends to which a second component called exoglucanase can then attach to release sugars from that chain. In fact, it releases mostly combinations of two sugar units called cellobiose into solution as the enzyme progresses along that chain. Cellobiose in turn is broken down by another enzyme component called beta-glucosidase to release single glucose molecules.
Biological routes have a number of potential advantages for the breakdown of biomass to support the production of fuels. First of all, they are highly selective, meaning that they form very few—if any—products other than those intended. In addition, they offer high yields that are critical to economic success for commodity products. There are also opportunities for entirely new organisms and enzymes through the ever evolving techniques of modern biotechnology. There is also substantial experience with the application of biological processing to conversion of starch and sugar into ethanol. In addition, the low temperatures and pressures required make containment relatively inexpensive, and they produce ethanol and other fuels that can replace gasoline. One disadvantage of biological processes is that they are very specific about the substrates they will attack, resulting in some materials being very difficult for them to breakdown. Also cellulosic biomass conversion is not yet commercial, and a lot of work has to be done to prove and apply the technology. In addition, the reactions are very slow. Thermochemical approaches, on the other hand, have a number of advantages including that they can handle a broad range of substrates and that the processes are very robust. There is also substantial commercial experience with thermochemical processes, for example, the Sasol process in South Africa that has been operating for decades converting coal to syngas for the production of diesel fuel substitutes. The reactions are fast and can produce products that can replace conventional fuels. Some of the major challenges facing thermochemical routes, however, include the requirement for very large operations to achieve economies of scale and to be economic, which requires extremely high capital investments. There is also less control of by-product formation from thermochemical processes, so there could be considerable challenges in dealing with some of the streams and waste products. Finally, pressures and temperatures tend to be high, presenting containment challenges.
A major need for producing commodity products is to achieve high yields. For example, for biomass costing $65 per ton, achieving a yield equivalent to 100 gallons of gasoline per ton would result in a feedstock cost of only 65 cents per gallon of gasoline equivalent. On the other hand, for half that yield, the cost of the feedstock per gallon would double in this particular case to $1.30 per gallon. Thus, the message here is that high yields are critical to economic success.
6.4 Laboratory Methods to Make Reactive Intermediates
A critical aspect to designing laboratory experiments is to decide what you are looking for in the particular experiments to be conducted. For example, it is often desirable to start by understanding reaction kinetics because the results tell you the potential to make the desired products. On the other hand, we also have to be concerned with effects of heat, mass, and momentum transfer on process scale-up as they can constrain achieving the desired products and yields. Generally, it is preferable to first establish reaction kinetics to determine the potential products that can be made and the maximum yields that are possible. Such kinetic experiments can be successfully carried out on a very small scale. On the other hand, consideration of transport impacts should be done in the context of a commercial design as we are trying to figure out what kind of effects would occur in real commercial equipment. Both present challenges due to the heterogeneity of biomass and the fact that we are dealing with multi-phase systems.
Another challenge to keep in mind is that the biofuels processes we are going to build are very, very large. For example, we can be processing of the order of 2,000 dry tons per day or more of biomass, and process units, therefore, can be quite large. Reactors for the pretreatment of biological cellulose prior to conversion can be of the order of six feet in diameter and over 40 feet long, and there may be several banks of such reactors. Commercial fermentors can be of the order of 500,000 to a million gallons each or so, while our experiments are run at the bench scale or, at best, in a pilot plant. Therefore, typically we are talking about scaling up from experience gained at perhaps a pilot plant with about one ton of biomass to between 1,000 and 2,000 or more tons per day, i.e., three orders of magnitude. The challenge of such a large scale up factor can make investors very nervous as they are concerned about extrapolating data over three orders of magnitude to arrive at a commercial design coupled with investments of the order of $300 million.
Against this background, the mission of the University of California at Riverside aqueous biomass processing research is first of all to improve the understanding of biomass fractionation, pretreatment, and cellulose hydrolysis to support applications and advances in biomass conversion technologies for the production of low cost commodity products. In addition, we seek to develop advanced technologies that would dramatically reduce the cost of production. To do this, graduate students, post-doctoral candidates, and research engineers on our team conduct such research. We also have developed extensive capabilities for biomass conversion, with particular focus on pretreatment of cellulosic biomass to open up its structure and release sugars followed by application of thermochemical or enzymatic processes to release sugars from the remaining solids for conversion to ethanol. We have developed equipment for conversion of biomass into furfural, levulinic acid, and formic acid, as reactive intermediates for catalytic processing to drop in fuels. Our equipment ranges in size from what we call a high throughput pretreatment and hydrolysis system, which can process of the order of 3 or 4 milligrams of biomass, up to our steam gun reactor that can process about a pound of biomass at a time. In addition to pretreatment capabilities, we have fermentors that allow us to ferment the sugars we release during pretreatment and hydrolysis to ethanol or other products including continuous trains of reactors.
6.5 Pretreatments and Biological Production of Sugars as Reactive
Intermediates Through the Consortium for
Applied Fundamentals and Innovation (CAFI)
A number of years ago, a team of researchers who had worked in biomass conversion for some time formed what we called the Biomass Refining Consortium for Applied Fundamentals and Innovation (CAFI) with the goal of better understanding different options for the pretreatment of biomass, followed by the production of sugars by enzymatic processing. This team focused on pretreatment to reduce the natural resistance of biomass to breakdown to sugars followed by a series of biological steps to make enzymes and then break down the polymers in biomass to form sugars. That was followed by fermentation to ethanol or other products with the residue that was left behind, primarily lignin, assumed to be burned to generate heat and power to run the process with excess power left for export. Techno-economic evaluations of this type of process have shown that the most expensive single component in the overall cost of the process is feedstock at about 1/3 of the total cost. However, that is quite low when we keep in mind that feedstocks for commodity products should typically represent of the order of 75 to perhaps 90% of the final product cost. The next biggest process cost was attributed to pretreatment at about 18% of the total. Therefore, working on improvements in pretreatment is critical to coming up with low cost biological processing. Other major costs were the biological conversion steps of making enzymes and using those enzymes for conversion of pretreated material to products, with those two together representing 21% of the total cost. Lesser costs were associated with such steps as distillation and solids recovery at about 10% of the total, waste water treatment at about 4%, and boiler turbo-generator at a net of about 4%; utilities and product storage were also relatively small cost contributors [7].
Pretreatment is critical in this entire operation with its role being to disrupt the orderly structure of biomass to open it up for access to enzymes that can in turn break down cellulose to release glucose sugar. Generally, pretreatment is done by the application of heat and potentially by the addition of chemicals. For example, pretreatment can be applied to break down hemicellulose to form sugars and disrupt lignin, and the result is cellulose that becomes exposed for enzyme action. When we look at the overall placement of pretreatment, it is pretty much in the center of the entire process and, therefore, has impacts on all surrounding operations. For example, the choice of pretreatment can affect the choice of feedstock and vice versa since not all pretreatments are capable of processing all feedstocks. In addition, pretreatment has an effect on the size reduction requirements as well as potentially on such aspects as harvesting and storage. Enzyme production is influenced by the choice of pretreatment as it determines solid characteristics that the enzymes must attack, and, therefore, the type of activities that are required from these enzymes. Another step impacted by pretreatment is downstream fermentations. For example, we must condition the liquid from pretreatment to make it less inhibitory to fermentation, and the choice of pretreatment has a major impact on the types of inhibitors and removal strategies employed. Similarly, we can show that pretreatment affects product recovery by determining the concentration of the final product and therefore recovery costs and the suitability of the final residues for biological waste treatment or other steps to utilize or dispose of those materials.
When we look at factors affecting enzymatic digestion of cellulose due to pretreatment, there are a number of substrate-related and enzyme-related factors to consider. Substrate-related factors include accessible surface area of the cellulose to enzymes, cellulose crystallinity, lignin and hemicellulose content and modification, the degree of polymerization of cellulose, particle size of the substrate, accessible bonds for breakdown, and deacetylation of biomass. Pretreatment impacts each of these factors. On the other hand, enzyme-related factors are such things as non-specific binding, end product inhibition, thermal inactivation, activity balance for synergism, specific activity, deactivation time, and enzyme immobility.
Numerous pretreatments have been studied to improve enzymatic digestion. These can be characterized in a number of ways. First of all, we can look at the type of additive; for example, none, acid, base, solvent, or enzymes. Also, we can look at the type of system: physical, chemical, thermal, or biological. Another consideration is whether pretreatment is operated in a batch, continuous, flow-
through, or counter-current mode. Solids concentration is another important consideration in pretreatment design as is heat-up method and heat-up time. Cool-down method and time must also be considered. Overall, numerous combinations of pretreatment devices have been trialed. In general, such approaches have been long on invention but short on developing fundamental knowledge of such pretreatment systems.
As mentioned, pretreatments can be classified into three major classes. Physical pretreatments are those that require only physical action such as size reduction to prepare material for biological conversion. In general such approaches tend to be very energy-intensive and do not achieve the high yields necessary. A second option is a biological approach which seeks to use enzymes to open up the structure of biomass to prepare it for subsequent downstream operations. However, such biological systems have been difficult to control and be effective. Finally, the third option is what we can call a thermochemical route in which the addition of chemicals is combined with heat to break down biomass and open up its structure. Most successful methods have fallen into the last category.
Pretreatment is faced with a number of important constraints on cost that must be taken into consideration during the development of such technologies. First of all, high yields are critical to distribute operating and capital costs over as much product as possible, and therefore minimize the unit costs for each. Low operating costs are essential to provide a margin for return on capital. This translates into low use of chemicals, energy, and labor. In addition, operating costs must be lower for the overall process than for competing technologies that generally have all their capital already paid for. Finally, low capital costs are essential to minimize exposure. For example, low cost containment meaning small vessel size, low pressures, and low temperatures are very desirable to keep capital costs low. Also, we want to have as few steps as possible to minimize capital costs.
Against this background, the Biomass Refining Consortium for Applied Fundamentals and Innovation (CAFI) was organized in late 1999 and early 2000 to better understand and develop pretreatment technology. The approach of the CAFI team was to employ common feedstocks, shared enzymes, identical analytical methods, same material and energy balance methods, and the same costing methods on leading pretreatment options to provide data that others can use to identify which technologies are best suited to their needs. The CAFI team also wanted to seek to understand mechanisms that influence performance and differentiate pretreatments. This would provide a technology base to facilitate commercial use. It would also facilitate identification of promising paths to advance pretreatment technologies.
Over the years, three different projects were funded for the CAFI team. The first focused on corn stover pretreatment by different methods. The second CAFI project focused on utilization of poplar wood and its conversion to sugars and the fermentation of sugars to ethanol. And the third CAFI project looked at the interaction of all the different steps surrounding pretreatment for application to switchgrass.
Over a period of ten years, the CAFI projects were guided by an Agricultural and Industrial Advisory Board consisting of representatives from about 26 different organizations. This Board met with the CAFI team twice a year to review the CAFI team’s progress and offer suggestions for improvements and new approaches. The CAFI technologies studies included ammonia recycle percolation and soaking aqueous ammonia by Y.Y. Lee at Auburn University, dilute active pretreatment by Charles Wyman at the University of California at Riverside,
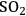 pretreatment by Jack Saddler at the University of British Columbia and Charles Wyman at the University of California at Riverside, ammonia fiber expansion (AFEX) by Bruce Dale at Michigan State University, controlled pH pretreatment by Michael Ladisch at Purdue University, and lime pretreatment by Mark Holtzapple at Texas A&M University. In addition, The National Renewable Energy Laboratory through Rick Elander provided logistical support and economic analysis for the CAFI team. Additionally, enzymes were provided by Genencor through Ryan Warner and feedstock by Ceres Corporation through Bonnie Hames and Steve Thomas.
pretreatment by Jack Saddler at the University of British Columbia and Charles Wyman at the University of California at Riverside, ammonia fiber expansion (AFEX) by Bruce Dale at Michigan State University, controlled pH pretreatment by Michael Ladisch at Purdue University, and lime pretreatment by Mark Holtzapple at Texas A&M University. In addition, The National Renewable Energy Laboratory through Rick Elander provided logistical support and economic analysis for the CAFI team. Additionally, enzymes were provided by Genencor through Ryan Warner and feedstock by Ceres Corporation through Bonnie Hames and Steve Thomas.
A key aspect of the CAFI project was the development of complete material balances for each pretreatment step. This involved tracking all the major components of biomass, primarily glucose, xylose, and lignin as the material went from size reduction to pretreatment to downstream conditioning and hydrolysis. A unique way to look at yields in the case of the CAFI project was to consider yields on the basis of total glucose plus xylose present in each feedstock and to determine what fraction of the total of those two sugars was released. This approach reflected the fact that most feedstocks are richer in glucose than xylose and to count them equally would not recognize the difference in economic impact.
The first CAFI project focused on corn stover, as mentioned earlier. This project was funded by the U.S. Department of Agriculture Initiative for Future Agriculture and Food Systems (IFAFS) Program through a competitive solicitation with the participation by the National Renewable Energy Laboratory (NREL) supported by additional funds from the DOE Office of the Biomass Program. This project began in September of 2000 and was completed in September of 2004 and found that all the different pretreatments had similar performance and costs. It is particularly noteworthy that when we compare the different pretreatments for corn stover, they all release similar amounts of glucose and xylose, and the major difference was just when such materials were released. For example, dilute acid pretreatment released most of the xylose during the pretreatment step and most of the glucose in the downstream enzymatic hydrolysis step. On the other hand, higher pH pretreatments such as lime would release a fair amount of the lignin as well as some xylose during pretreatment, and the bulk of the glucose during enzymatic hydrolysis. AFEX was unique among the different pretreatments in that it released virtually nothing during pretreatment but made the biomass very accessible to enzymes for a high yield production of sugars downstream.
The second CAFI project started in April of 2004 through funds from the Department of Energy Office of Biomass Program through a competitive solicitation. In this particular project the CAFI team determined more in-depth information on enzymatic hydrolysis of hemicellulose and cellulose in the solid following pretreatment, and also conditioning and fermentation of the hydrolysis liquids. In addition, the University of British Columbia was added to the team to work on sulfur dioxide pretreatment through support of Natural Resources Canada. Again, Genencor supplied commercial and advanced enzymes for the project. Greater differences were found among the different pretreatment technologies for poplar with the highest yields from sulfur dioxide and lime approaches.
The third CAFI project focused on switchgrass as a feedstock. Again, switchgrass was pretreated by all the leading technologies, and material balances were closed by common methods for each pretreatment. Sugar yields were determined versus cellulase enzyme loadings, and the benefits of adding different enzyme activities such as beta-glucosidase and xylanase were evaluated. The CAFI was also able to characterize the effects of key enzyme features and surface characteristics on performance. Furthermore, the effect of switchgrass age, harvest time, and location was explored for the different pretreatments coupled with enzymatic hydrolysis. Three different types of switchgrass were used: one called Alamo, another called Shawnee, and a third known as Dacotah. They were quite similar in many respects, although the Dacotah switchgrass had a higher lignin content and lower free sugars than the other two, primarily due to the Dacotah switchgrass being harvested in the late winter/early spring while the other two were harvested in the fall. In this case, performance was intermediate between that for corn stover and poplar with lime and sulfur dioxide pretreatments achieving the best yields. However, all did reasonably well with switchgrass. In these studies, a wide range of conditions were applied for different pretreatments.
Overall, the different pretreatments have different effects on the substrate. The lowest pH pretreatments with dilute acid or
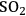 remove most of the hemicellulose as monomers and remove low amounts of lignin. At near neutral pH by controlled pH pretreatments with hot water, hemicellulose was hydrolyzed to mostly oligomers in solution, and a limited amount of lignin was removed. Further up the pH scale, ammonia fiber expansion removed almost nothing as noted earlier but opened up the structure for high yield release of sugars by enzymes. Finally lime or soaking with aqueous ammonia pretreatments removed more lignin than hemicellulose and left much of the carbohydrates in the solids.
remove most of the hemicellulose as monomers and remove low amounts of lignin. At near neutral pH by controlled pH pretreatments with hot water, hemicellulose was hydrolyzed to mostly oligomers in solution, and a limited amount of lignin was removed. Further up the pH scale, ammonia fiber expansion removed almost nothing as noted earlier but opened up the structure for high yield release of sugars by enzymes. Finally lime or soaking with aqueous ammonia pretreatments removed more lignin than hemicellulose and left much of the carbohydrates in the solids.
Key messages from the CAFI project were that first of all it is very important to have transparent material balances to facilitate comparison among different technologies. Also it is clear that pretreatment is still required to achieve high yields from all three substrates; corn stover, poplar and switchgrass. The choice of pretreatment will also depend on interactions with the rest of the process, such as the type of enzymes used and their activities. CAFI also found that not all pretreatments were equally effective for all feedstocks, and some feedstocks favored certain pretreatments over others. Also, the choice of enzyme formulation and pretreatment technology are linked, and the type of activities needed for enzymes depends upon the characteristics of the solids from pretreatment. In addition, feedstock variability can have a large impact on performance, but the cause and effect between pretreatment alteration of feedstock and enzymatic digestion is not entirely clear. Enzyme loadings are still higher than desired for economic reasons for all the pretreatments, so continued work is needed on pretreatment to find approaches than can reduce enzyme requirements. Hopefully, these results will help others select pretreatment, feedstock, and enzyme combinations that are effective for commercial use.
It is important to note that the results of the CAFI team have been published widely in various journals, with one dedicated volume of Bioresource Technology devoted to reporting the CAFI I results for corn stover in 2005 [8]. Another special volume in Biotechnology Progress reported the CAFI results with poplar in 2009 [9] . Finally, the CAFI team published papers for a targeted volume in Bioresource Technology where application of all these different pretreatments to switchgrass was presented in a single volume. At this point, the CAFI project has concluded, and there are no plans to continue [10].
6.6 Thermochemical Processing to Sugars and
Other Reactive Intermediates
Key objectives for biomass pretreatment are to capture a large fraction of fermentable hemicellulose sugars to realize high ethanol yields and to minimize formation of degradation products, to minimize inhibition and detoxification needs. It is also critical to realize high yields of glucose from cellulose in pretreatment and enzymatic hydrolysis. In general, dilute acid catalyzes breakdown of hemicellulose to form oligomers which in turn form sugar monomers by that acid as well. However, continued holding of xylose in the presence of dilute acid at high temperatures forms furfural and degradation products. Thus, this sequence represents a classical series reaction, with xylose being the intermediate product between the breakdown of biomass to form oligomers and their breakdown to xylose followed by the breakdown of xylose to furfural and on to degradation products. As a result, sugar generation must be balanced against sugar degradation to maximize yields. Fortunately, in the presence of dilute sulfuric acid at temperatures on the order of 160 to 170 °C or so, we can achieve xylose yields of about 90% before degradation becomes a problem.
In a similar way, cellulose hydrolysis is also catalyzed by dilute acid to form primarily glucose which in turn will breakdown through dehydration to form HMF followed by further dehydration to levulinic acid followed by degradation products. In this case, however, it is much more challenging to obtain high yields that we see for hemicellulose hydrolysis due to the crystalline structure and other aspects of cellulose composition. For example, typically we see glucose yields of the order of 50%, and residence times are only for a few seconds at very high temperatures of the order of 240 °C in the presence of around 1% sulfuric acid to achieve such yields. Unfortunately, it is very difficult to control conditions so precisely in commercial scale equipment to obtain that performance.
However, further consideration of these reactions shows that products formed by breakdown or dehydration of xylose and glucose can be useful for catalytic conversion to other products. For example in the case of xylose, dehydration of xylose in the presence of dilute acid at moderate temperatures of around 160 °C forms furfural, and furfural can be catalytically reacted to form hydrocarbon products. Similarly, holding glucose at high temperatures in the presence of dilute acid forms HMF, which in turn breaks down to levulinic and formic acids which can in turn be converted into hydrocarbon fuels [10].
6.7 Conclusions
Biomass is a unique resource for sustainable production of liquid fuels that we particularly favor to power transportation. Cellulosic biomass offers the low costs and abundance essential to make a meaningful impact on fuel use. A variety of thermal and biological processes can be applied to convert biomass into fuels. However, the natural resistance of cellulosic biomass to breakdown to reactive compounds must be overcome to achieve low costs. Aqueous processing of cellulosic biomass can produce sugars that can be biologically fermented into fuels such as ethanol as well as various other products. In this case, the power of modern biotechnology offers the potential for very low costs. However, aqueous processing can also produce sugar dehydration products such as furfural, HMF, and levulinic acid that can be catalytically reacted to hydrocarbon fuels that are compatible with our existing infrastructure. Thus, aqueous processing of cellulosic biomass offers a versatile route to support low cost production of liquid fuels for transportation and other applications.
Acknowledgments
Support of the Ford Motor Company for the Chair in Environmental Engineering at the Center for Environmental Research and Technology of the Bourns College of Engineering at the University of California, Riverside is gratefully acknowledged for making research such as described possible. We also thank the BioEnergy Science Center (BESC), a U.S. Department of Energy Bioenergy Research Center supported by the Biological and Environmental Research Office in the DOE Office of Science, for supporting our research. Other agencies that have made our research possible include the Defense Advanced Research Projects Agency (DARPA) through subcontracts by Logos Technologies and the University of Massachusetts, Amherst; the USDA National Research Initiative Competitive Grants Program, contract 2008-35504-04596; the US Department of Energy Office of the Biomass Program, contract DE-FG36-07GO17102. Finally, it is important to recognize the numerous past and present students, coworkers, and partners who make our research possible.
Bibliography
[1] U.S. Department Energy () Annual Energy Review 2009.
[2] L.R. Lynd. Overview and Evaluation of Fuel Ethanol from Cellulosic Bio- Annual Reviews of Energy and Environment 21: 403-465 (1996): 403-465.
[3] R. Perlack, L. Wright, L. W., Turhollow L., T. L., A. Turhollow. Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply.. Washington, DC: Energy Information Administration, 2005
[4] L.R. Lynd, C.E. Wyman, C. W.. Biocommodity Engineering Biotechnology Progress 15: 777-793 (1999): 777-793.
[5] G.W. Huber, S. Iborra, S. I.. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering Chemical Reviews 106: 4044-4098 (2006): 4044-4098.
[6] C.E. Wyman, S.R. Decker, S. D., Himmel S.R., H. S., M.E. Himmel. Hydrolysis of Cellulose and Hemicellulose. In: Polysaccharides: Structural Diversity and Functional Versatility Ed. by S. Dumitriu. New York: Marcel Dekker, Inc., 2004. 995-1033.
[7] A. Aden, M. Ruth, M. R.. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing co-current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover: National Renewable Energy Laboratory. Golden, CO: National Renewable Energy Laboratory, 2002
[8] C.E. Wyman, B.E. Dale, B. D., Elander B.E., E. B., R.T. Elander. Comparative Sugar Recovery Data from Laboratory Scale Application of Leading Pretreatment Technologies to Corn Stover Bioresource Technology 96: 2026-2032 (2005): 2026-2032.
[9] C. Wyman, B. Dale, B. D., Elander B., E. B., R. Elander, R. E., Holtzapple R.. Comparative Sugar Recovery and Fermentation Data Following Pretreatment of Poplar Wood by Leading Technologies. Biotechnology Progress 25: 333-339 (2008): 333-339.
[10] C.E. Wyman, V. Balan, V. B., Dale V., D. V., B.E. Dale, B. D., Elander B.E., E. B., R.T. Elander, R. E., Falls R.T., F. R.. Comparative Data on Effects of Leading Pretreatments and Enzyme Loadings and Formulations on Sugar Yields from Different Switchgrass Sources Bioresource Technology 102(24): 11052-11062 (2011): 11052-11062.
